On Nov. 1, the Centers for Medicare and Medicaid Services (CMS) released the 2025 Medicare Physician Fee Schedule final rule (Final Rule), addressing, among other things:
- Medicare payments: Overall, the 2025 reimbursement rates for allergy services will decrease. The College developed a spreadsheet that compares the 2024 reimbursement and Relative Value Units (RVUs) for allergy codes to the ones for 2025. (Note that these amounts do not account for geographic adjustments.)
- Flexibility for direct supervision: Through Dec. 31, 2025, CMS will continue to define direct supervision to allow real-time audio and visual interactive telecommunications. In addition, the agency will permanently define “direct supervision” to include audio-video communications technology for a subset of services related to allergy.
- Office/Outpatient evaluation and management (E/M) visits: CMS will allow payment of HCPCS code G2211 when the office/outpatient E/M base code (CPT 99202-99205, 99211-99215) is reported by the same practitioner on the same day as an annual wellness visit, vaccine administration, or any Medicare Part B preventive service.
- Telehealth: Certain Medicare telehealth flexibilities related to the COVID-19 public health emergency are expiring.
- Definition of refundable single-dose container or single-use package drug: CMS finalized its proposal to amend the definition of “refundable single-dose container or single-use package drug” to “single-patient-use container” as a package type term.
- Overpayment: Under circumstances where additional time is needed to investigate or calculate overpayments for Medicare Parts A and B, CMS will allow suspension of the existing 60-day timeline.
This article summarizes key proposals concerning the Medicare Physician Fee Schedule that impact allergy practices. For more details regarding changes to the traditional Merit-based Incentive Payment System (MIPS) program for the 2025 performance period, please refer to the Insider article.
Cuts to Medicare reimbursement
CMS reduced the conversion factor (CF) for 2025 to $32.35, a 2.83% decrease from the 2024 CF of $33.29. The application of the CF (along with other structural cuts) will drag down Medicare reimbursement levels for all practitioners, including allergists. The College opposed the reduction, and the College will continue to work with Congress to address these cuts to Medicare reimbursement rates.
The AMA graphic below depicts the decline in Medicare physician payment since 2020 and highlights the urgent need for congressional intervention before Jan. 1.
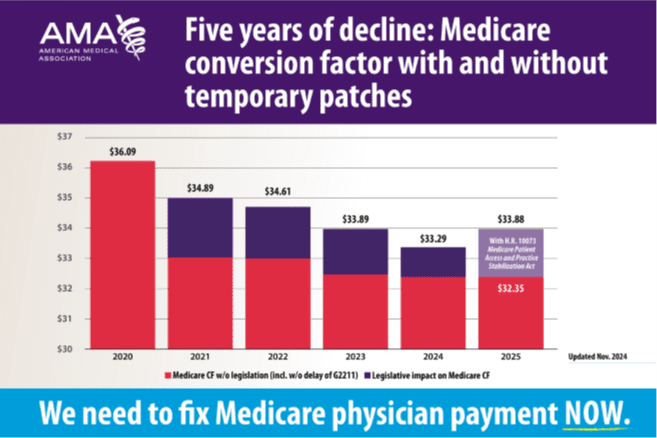
Source: Medicare Conversion Factor, 2020-2025
Direct Supervision
Medicare rules generally require that a physician be immediately available on site if the physician uses clinical staff to aid in the furnishing of a service. This principle is known as direct supervision. CMS temporarily relaxed this direct supervision requirement in response to the COVID-19 public health emergency, allowing the supervising physician/practitioner to be “immediately available” through virtual using two-way, real-time audio/visual technology. Thanks to the advocacy of the College’s Advocacy Council and other medical societies, CMS has decided to extend this policy through Dec. 31, 2025.
After Dec. 31, 2025, the agency will permanently allow this virtual presence for direct supervision for the following subset of services:
- CPT code 99211 (Office or other outpatient visit for the evaluation and management of an established patient that may not require the presence of a physician or other qualified health care professional).
- Services furnished incident to a physician or other practitioner’s service when provided by auxiliary personnel employed by the billing practitioner and working under their direct supervision, and for which the HCPCS code has been assigned a Professional Component (PC)/Technical Component (TC) indicator of 5. This includes CPT codes 95012 (Exhaled nitric oxide meas), 95044 (Allergy patch tests), 95052 (Photo patch test), 95056 (Photosensitivity tests), 95115 (Immunotherapy one injection), and 95117 (Immunotherapy injections).
Office/Outpatient E/M visits
CMS previously finalized separate payment for HCPCS code G2211 (Visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious condition or a complex condition. Add-on code, list separately in addition to office/outpatient evaluation and management visit, new or established). In the Final Rule, CMS finalized a policy to allow payment of HCPCS code G2211 when the base CPT codes 99202-99205, and 99211-99215 are reported by the same practitioner on the same day as an annual wellness visit, vaccine administration, or any Medicare Part B preventive service. However, G2211 cannot be billed when modifier -25 is used, i.e., for an office visit and allergy testing or an office visit and spirometry.
Telehealth
Beginning in 2025, the patient’s home is a permissible originating site only for services for the diagnosis, evaluation, or treatment of a mental health or substance use disorder, and for the monthly ESRD-related clinical assessments. Other patients will generally need to be present at a medical facility during telehealth visits.
Through 2025, CMS will continue to permit the distant site practitioner to use their currently enrolled practice location instead of their home address when providing telehealth services from their home. The College supported this policy to help ensure the privacy and safety of physicians.
Requiring manufacturers of certain single-dose container or single-use package drugs to provide refunds with respect to discarded amounts
Current law requires drug manufacturers to provide a refund to CMS for certain discarded amounts from a refundable single-dose container or single-use package drug (hereinafter referred to as “refundable drug”). In the 2025 Final Rule, CMS finalized its proposal to amend the definition of “refundable single-dose container or single-use package drug” to “single-patient-use container” as a package type term. CMS added three types of products that may be considered refundable single-dose container or single-use package drugs. These are:
(1) Product furnished from a single-dose container or single-use package based on FDA-approved labeling or product information.
(2) Product furnished from an ampule for which product labeling does not have discard statement or language indicating the package type term, like “single-dose container,” “single-use package,” “multiple-dose container,” or “single-patient-use container.”
(3) Product furnished from a container with a total labeled volume 2 ml or less for which product labeling does not have language indicating the package type term, like “single-dose container,” “single-use package,” “multiple-dose container,” or “single-patient-use container.”
Overpayment
Under circumstances where additional time is needed to investigate or calculate overpayments for Medicare Parts A and B, CMS will allow suspension of the existing 60-day timeline. Rather, providers and practitioners would be required to report and return overpayments within the sooner of 180 days after identifying the overpayment, or after calculating overpayment aggregates upon conclusion of the investigation.
Read more information on the 2025 Medicare Physician Fee Schedule Final Rule.
The Advocacy Council – ADVOCATING FOR ALLERGISTS AND THEIR PATIENTS.




