Beginning July 1, Medicare will require the use of the new JZ modifier for single-dose containers when there are no discarded amounts of a drug/biologic. The JW modifier continues to be required when not all of all the drug/biologic in a single-dose container is used.
| Modifier | Short Descriptor | Long Descriptor |
|---|---|---|
| JW | Discarded drug not administered | Drug amount discarded/not administered to any patient |
| JZ | Zero drug wasted | Zero drug amount discarded/not administered to any patient |
In general, the JW and JZ modifier policy applies to all drugs separately payable under Medicare Part B that are described as being supplied in a “single-dose” container or “single-use” package based on FDA-approved labeling.
| 2023 Implementation Timeline to the Discarded Drug Unit Modifiers Policy | ||
|---|---|---|
| START REPORTING | REQUIRED REPORTING | |
| Jan. 1 – June 30, 2023 | July 1 | October 1 |
|
Effective Jan. 1, 2023, the updated discarded drugs and biologicals policy now includes a new JZ modifier. The new JZ modifier may be reported between Jan. 1, 2023 and June 30, 2023. The JW modifier has been required since Jan. 1, 2017. |
Effective July 1, 2023, providers are required to report the JZ modifier to attest no discarded units. Claims that bill for drugs furnished on or after July 1, 2023 that do not report the JW or JZ modifier may be subject to provider audits. |
Starting Oct. 1, 2023, claims that do not use the modifiers as appropriate may be returned as unprocessable until claims are properly resubmitted. |
The use of these modifiers is not appropriate for drugs that are from multiple-dose containers. This includes a drug or biologic in which one National Drug Code (NDC) assigned to its billing code is a multiple-dose container and other NDCs assigned to the same billing code are also single-dose containers.
The JW and JZ modifier policy applies to all providers and suppliers who buy and bill separately payable drugs and biologics under Medicare Part B. The modifiers are required in the physician office setting, hospital outpatient setting, in critical access hospitals and 340B hospitals.
Claims that bill for separately payable drugs under Part B from single-dose containers that don’t report the JW or JZ modifier on or after July 1, 2023, may be subject to audits.
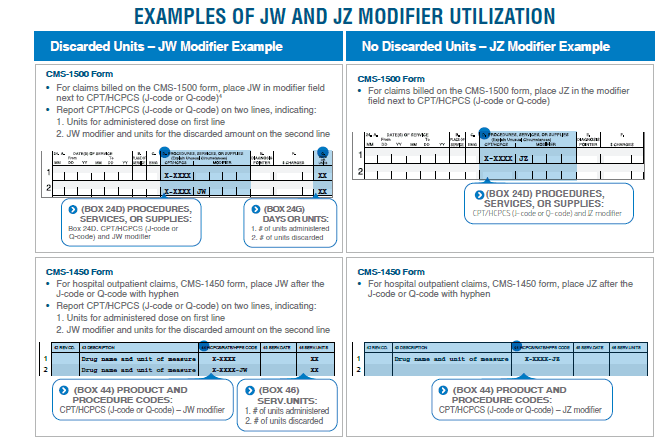
For further details about the JW and JZ modifiers, consult the CMS’ Discarded Drugs and Biologicals JW-JZ Modifier FAQs.
The Advocacy Council – ADVOCATING FOR ALLERGISTS AND THEIR PATIENTS.



