Allergy practices must comply with USP 797 – Section 21 – the Allergy Exception – beginning Nov. 1, 2023. Section 21 applies to physicians mixing patient specific allergen extracts in their offices for subcutaneous immunotherapy. The remainder of USP 797 does not apply.
As early as 2007, USP included an allergy exception in its rule. However, after the fungal meningitis outbreak in 2012, where more than 100 patients died, Congress began scrutinizing compounding centers. The Drug Quality and Security Act became law and the FDA increased oversight over compounding pharmacies.
Through our Washington advocacy, an amendment was introduced by Rep H. Morgan Griffith (VA) to allow compounding under USP guidelines by allergists and other physicians who mixed drug/biologics in limited quantities for an identified individual patient.
The 2015 draft revision of USP omitted Section 21 and threatened the continuation of allergists’ ability to mix extracts in their offices. Allergy leaders – Drs. Mike Nelson, Tom Casale, Steve Kagan, and Jim Sublett – met with USP to convince them that the process allergists followed in preparing allergenic extracts was not the same as the compounding done by pharmaceutical companies. Allergen Extracts are recognized as unique. In order for USP to continue with the allergy exception, our leaders promised to educate allergists in proper mixing techniques. Ensure your individual office staff and mixing areas are compliant with USP 797 requirements.
USP revised Chapter 797 again and published a revised version in 2019. Minimal “wordsmithing” was done, and the final version was approved in 2022 with an implementation date of Nov. 1, 2023. The latest USP document can be confusing and unfortunately, some misinformation is circulating. Our Allergen Extract Mixing Toolkit contains all the resources you need to be compliant with the new rule. You can also access the full USP 797 rule at USP’s website, but it does require a paid subscription.
Reporting compliance with specific individual requirements of Chapter 797 on each insurance claim should not be a requirement for payment for providing allergy services.
Read below for the requirements of Section 21 and some important highlights. Federal and State laws require compliance with USP standards, and most state pharmacy boards defer to USP 797. In addition, the Joint Commission expects compliance with USP guidelines. Full details are included in Section 21.
21.1 Personnel Qualifications for Compounding
Important rule: If a member of the mixing team does not compound for six months, he/she must re-do all the annual training.
21.2 Personnel Hygiene and Garbing for Compounding
21.3 Facilities for Compounding
There are two options – compounding allergen extracts must occur in either an allergenic extract compounding area (AECA) OR an ISO Class 5 primary engineering control (PEC).
| Dedicated Allergenic Extracts Compounding Area (AECA) | ISO Class 5 Primary Engineering Control (PEC) |
|
|
|
|
|
|
|
|
|
A PEC is so much more than just having a laminar flow hood. A hood is only one component of the ISO Class 5 PEC. The requirements for an ISO Class 5 PEC are quite extensive and are detailed in section 4 of USP 797. Additionally, the PEC certification requirement, detailed in section 5 of USP 797, must occur initially and at least every six months. It includes not only the hood but the entire room. A laminar flow hood is not a requirement of an AECA.
We recommend you review the PEC requirements carefully before choosing to implement a PEC. Because the PEC requirements are so onerous, the College fought for and got USP approval for the AECA option for our specialty. The College believes the AECA will be a better option for almost all allergy practices.
Requirements for both PEC and AECA:
- Away from unsealed windows, doors that open to the outside.
- Restricted traffic and no other activities in the area while compounding.
- Neither a PEC nor an AECA may be located where environmental control challenges (e.g., restrooms, warehouses, or food preparation areas) could negatively affect the air quality.
- The PEC or the work surfaces in the AECA must be located at least 1 meter away from a sink.
21.4 Cleaning and Disinfecting
21.5 Establishing Beyond Use Dates (BUDs)
- The BUD for the prescription set must be no later than the earliest expiration date of any allergenic extract or any diluent that is part of the prescription set, and the BUD must not exceed one year from the date the prescription set is mixed or diluted.
- Example:
Vial being made on 7/19/2023: 1 year = 7/18/2024
Dust mite Expiration date 6/18/2024
Diluent Expiration date 12/1/2023
BUD 12/1/2023
21.6 Labeling
USP 797 requires the following information be displayed prominently and understandably on each vial of an allergenic extract:
- Patient name
- Type and fractional dilution of each vial, with corresponding vial number
- BUD
- Storage conditions
21.7 Shipping and Transport
- Inappropriate transport can adversely affect the quality of allergenic extract prescription sets.
- Must select modes of transport that are expected to deliver properly packed prescription sets in an undamaged, sterile, and stable condition.
- If sets require special handling, must include specific handling instructions on the exterior of the container.
21.8 Documentation
All facilities where allergenic extract prescription sets are prepared must have and maintain written or electronic documentation to include, but is not limited to, the following:
- SOPs describing all aspects of the compounding process.
- Personnel training records, competency assessments, and qualification records including corrective actions for any failures.
- Certification reports of the PEC, if used, including corrective actions for any failures.
- Temperature logs for refrigerator(s).
- CRs for individual allergenic extract prescription sets.
Compounding records / log must include the following:
- Name, strength or activity, and dosage form of the CSP.
- Date and time of preparation of the CSP.
- Assigned internal identification number (e.g., prescription, order, or lot number).
- A method to identify the individuals involved in the compounding process and individuals verifying the final CSP.
- Name of each component.
- Vendor, lot number, and expiration date for each component for CSPs prepared for more than one patient (Mixes; trees, weeds…).
- Volume of each component.
- Strength or activity of each component.
- Total quantity compounded.
- Final yield (e.g., quantity, containers, number of units).
- Assigned BUD and storage requirements.
- Results of QC procedures (e.g., visual inspection, filter integrity testing, pH testing).
- If applicable, the CR must also include: MFR reference for the CSP.
RESOURCES
- Allergen Extract Mixing Toolkit – Includes instructions, staff competency assessments, our extract preparation guide, checklists and links to webinars and our extract mixing quiz.
- Our exclusive, easy-to-read summary of the USP 797 Allergen Extract exception.
- The College’s USP 797 FAQs answers common questions about compliance, documentation and more.
- You can also access the full USP 797 rule at USP’s website – but it does require a paid subscription.
TESTING SUPPLIES
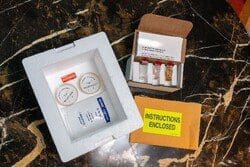
Everything you need shipped in a temperature -controlled cooler that includes:
- Media fill kit(s).
- Gloved fingertip-thumb sampling plates.
- Detailed instructions.
- Incubation and reporting services.
Receive a detailed report with the results to demonstrate your adherence with USP.
College members receive a 15% discount. Order today!
Back by popular demand! Our hands-on Allergy Mixing Workshop – April 20, 2024. Mark your calendars and watch the College Insider for more information.
The Advocacy Council – ADVOCATING FOR ALLERGISTS AND THEIR PATIENTS.




